Premium Methylene Blue for Professionals
Carefully crafted for precision. Trusted by researchers, chemists, and industrial labs.
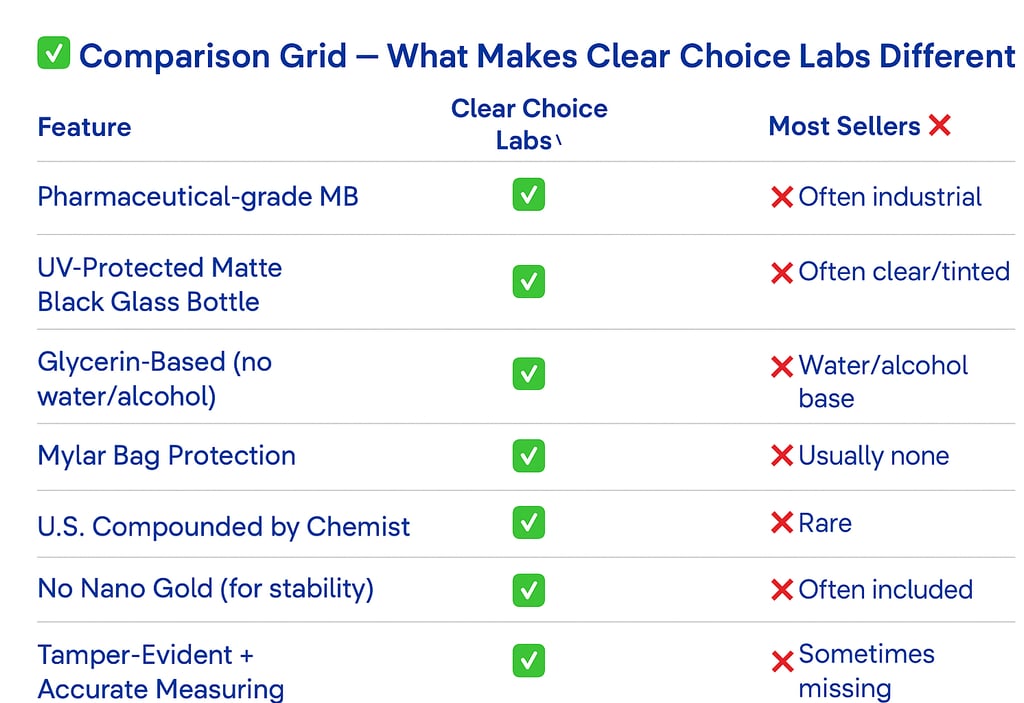
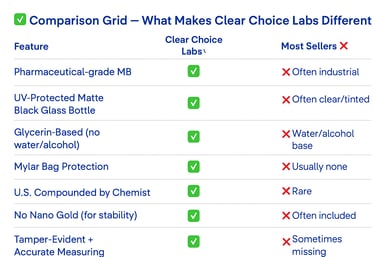
Why Our Methylene Blue Is the Clear Choice
Not all methylene blue is created equal. We took zero shortcuts in formulating, sourcing, packaging, and protecting ours — and we’ll show you why it matters..
🔬 Section 1: Most Methylene Blue Is Already Degraded
Most MB Manufacturers don’t understand how fragile methylene blue really is.
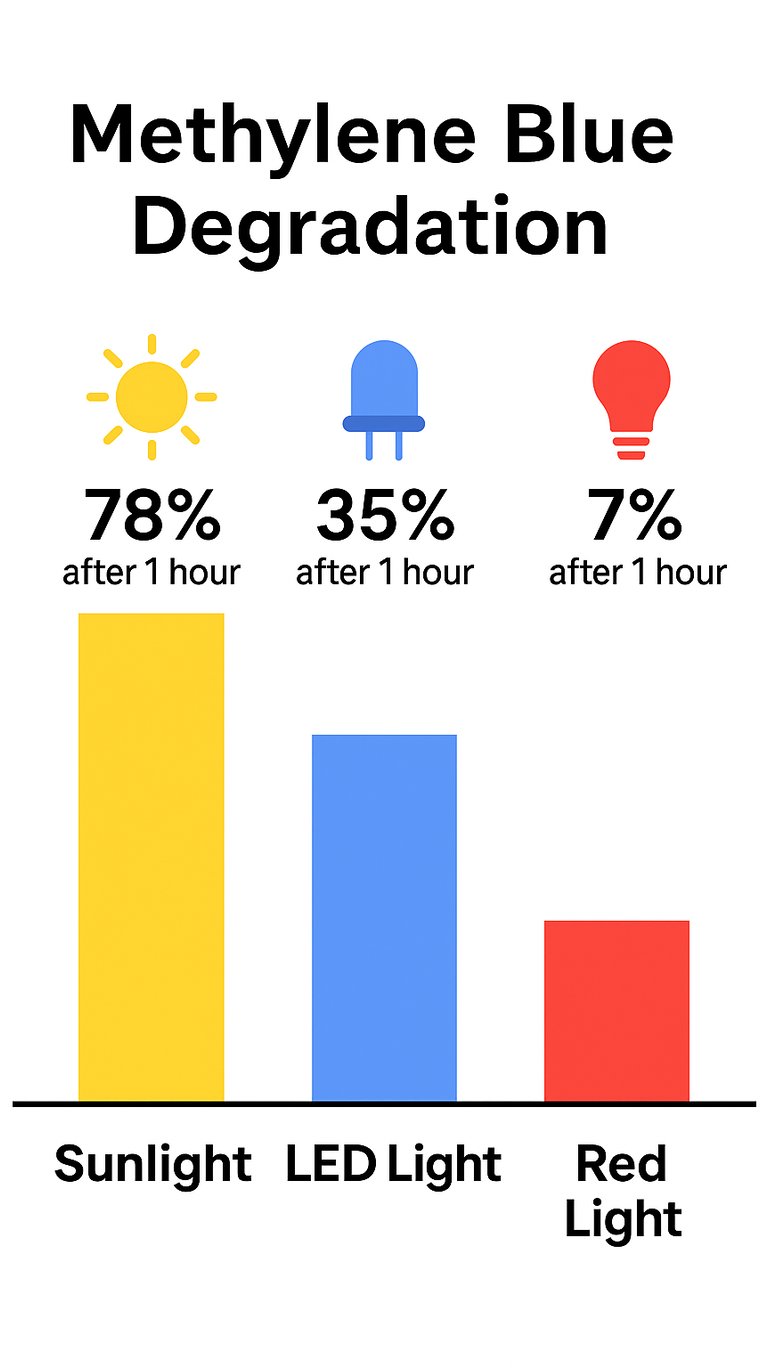
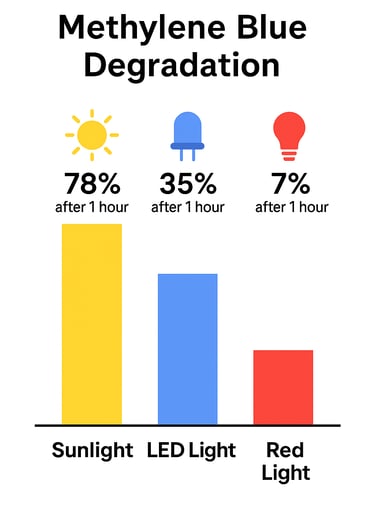
Methylene blue is extremely sensitive to light — and we’re not talking about slow, long-term degradation. Studies show methylene blue can begin degrading within minutes of UV exposure. When exposed to sunlight or even strong indoor lighting, the compound breaks down rapidly, losing potency and forming unwanted byproducts that compromise both purity and effectiveness.
Most sellers overlook this entirely.
They package methylene blue in clear or lightly tinted bottles, mistakenly believing that as long as the bottle isn't in direct sunlight, it's protected. But it’s not. Ambient indoor lighting — especially from windows or overhead bulbs — can still trigger degradation in minutes.
Unless a bottle is specifically engineered to block UV and blue light — like the custom fully opaque, Euro-style matte black bottles we use — your methylene blue will degrade. Once light hits it, the clock starts ticking.
That’s why our packaging was designed from the ground up to protect the compound at every stage:
Pharmaceutical-grade raw powder from a manufacturer that protects against light exposure during production and shipping
A fully opaque, UV-blocking glass bottle that prevents any light infiltration
A matte black exterior finish to stop scattering and ambient light
An additional black mylar impulse-sealed bag to block light during shipping and storage
If your methylene blue isn’t shielded like this, it’s already compromised.
💧 Section 2: Solvent Choice Makes or Breaks Stability
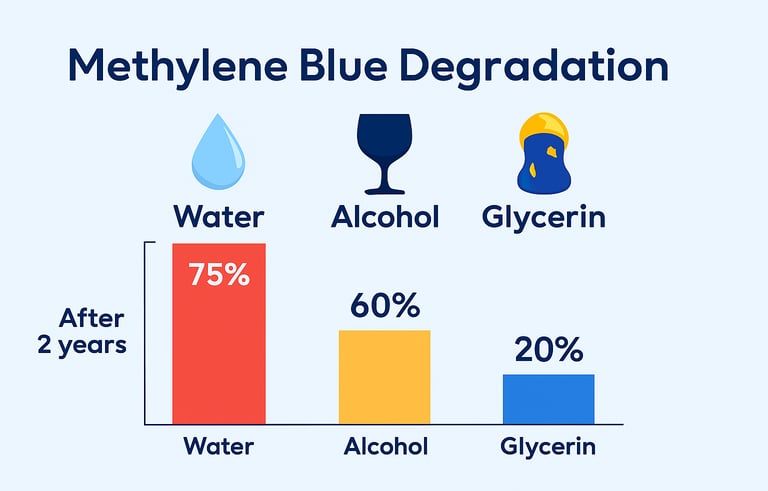
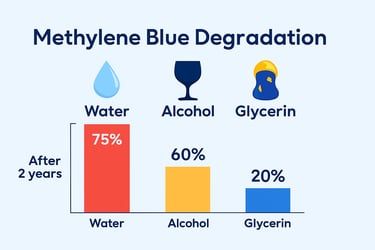
Water and alcohol significantly reduce shelf life. We chose glycerin.
Most methylene blue is suspended in water or alcohol — but both increase volatility and accelerate chemical breakdown. We use USP pharmaceutical-grade glycerin, a stable, inert base that cushions the compound, protects its structure, and extends usability.
We further stabilize the formula using citric acid to gently buffer pH, and potassium sorbate as a preservative to reduce oxidation and microbial risk.
🇮🇳 Section 3: Why We Chose Our India Manufacturer — and Rejected Dozens
We spent months sourcing the best.
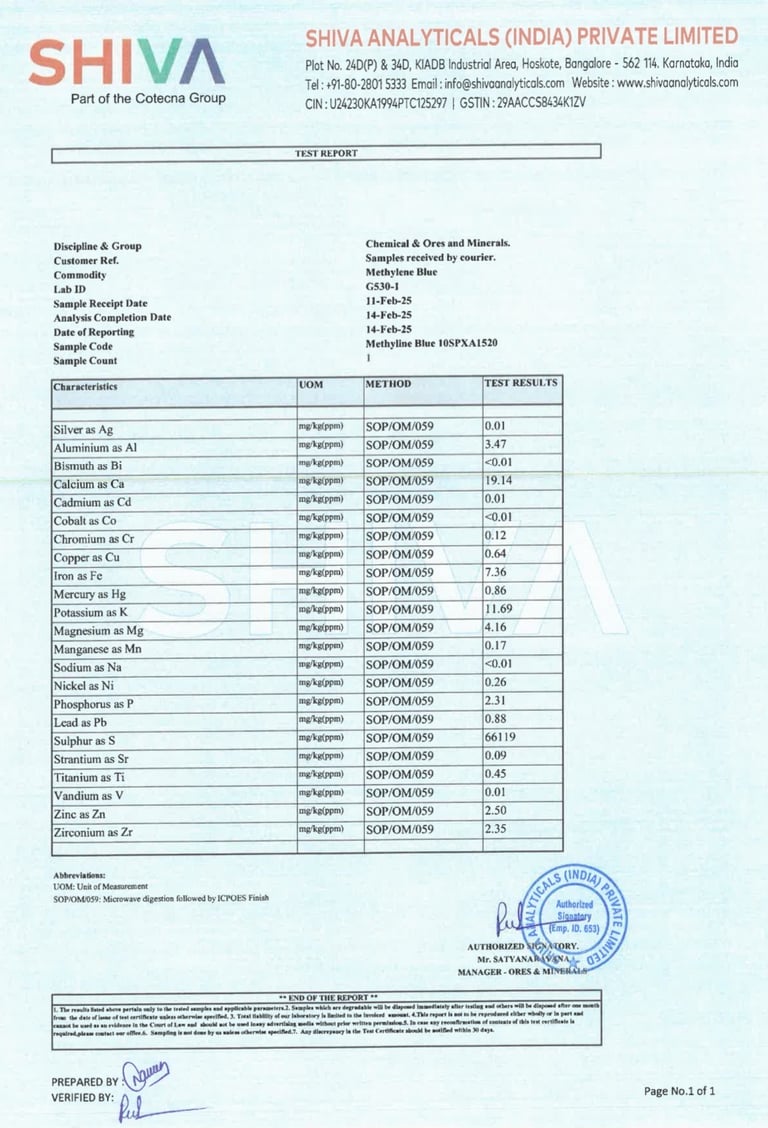
India is one of the world’s largest producers of fine chemicals and active pharmaceutical ingredients (APIs). Our supplier has spent over 70 years specializing in methylene blue alone. Their facility follows strict GMP standards, their COAs are clean and consistent, and they understand how to protect MB during manufacturing, handling, and shipment.
We rejected suppliers who couldn’t meet our standards — even when it meant delaying production.


👨🔬 Section 4: Chemical Suspension — Compounded in the USA
Our methylene blue isn’t just repackaged — it’s formulated and suspended in the U.S. under the direction of a chemist with over 20 years of experience working with delicate, high-purity compounds.
Our lead chemist was one of the first in the U.S. to pioneer advanced suspension techniques for sensitive compounds and has built a career around stabilizing difficult-to-formulate substances. Every decision — from solvent choice to pH balance — is made with long-term chemical integrity in mind.
🧪 Section 5: Our Process — Step by Step
Every bottle is formulated with purpose:
✅ Pharmaceutical-grade methylene blue — from a 70-year veteran chemical lab in India
✅ USP-grade glycerin — a clean, stable carrier (no alcohol, no water)
✅ Food-grade Citric acid + potassium sorbate — pH-balanced and preserved
✅ Matte black UV-blocking glass bottles — zero light infiltration
✅ Calibrated glass pipettes — accurate, consistent measurements
✅ Tamper-evident caps + mylar impulse-sealed pouches — maximum protection
No shortcuts. No fillers. Just the best raw materials, carefully compounded and protected for long-term reliability.
🪠 Section 6: Why We Don’t Add Nano Gold — and Why That’s Better
Some brands add "nano liquid gold" to their methylene blue — often for marketing. But while it sounds impressive, the science doesn’t back the benefit — and it can actually reduce shelf life.
Gold nanoparticles are delicate. They can:
❌ Clump or destabilize in the presence of citric acid or methylene blue
❌ Accelerate light-triggered breakdown if not perfectly shielded
❌ Shorten the lifespan of the entire formula if pH isn't tightly controlled
There’s no validated evidence that nano gold improves how methylene blue functions in a suspension — and without precise lab controls, the risk of instability outweighs any potential advantage.
We chose to leave it out — so you get a cleaner, more stable, and more scientifically supported product.
Real stability. No gimmicks. Just pure, potent methylene blue.
🧠 Section 7: Why It Matters
Methylene blue is powerful — but only if it’s handled with care.
The truth is, most methylene blue breaks down before it even reaches the customer. Weak packaging, unstable solvents, and rushed manufacturing often result in degraded, ineffective product.
At Clear Choice Labs, we designed every step of our formula to protect integrity and consistency from start to finish.
We focus on:
✅ Pharmaceutical-grade purity — from trusted GMP suppliers
✅ Stable, solvent-safe formulation — no water or alcohol
✅ Light-protected, precisely measured — for maximum reliability
✅ Verified consistency — every single time
Your standards are high. Ours are higher.
That’s why we don’t cut corners — we seal them.
Choose confidence. Choose clarity. Choose Clear Choice Labs.




📚 References
Wainwright, M. (2001). "Methylene blue derivatives—suitable photoantimicrobials for blood product disinfection?" International Journal of Antimicrobial Agents.
Aeschbacher, M., et al. (2012). "Degradation kinetics of methylene blue under various lighting conditions." Environmental Science & Technology.
Sabeena Farvin, K.H., et al. (2009). "Effect of solvents on stability of pharmaceutical suspensions." Journal of Pharmacy Research.
U.S. Pharmacopeia. "Glycerin Monograph."
India Brand Equity Foundation (IBEF). (2021). "India: The Pharmacy of the World."
World Health Organization. "Good Manufacturing Practices (GMP) Guidelines."
Allen, L.V. et al. (2004). "Stability of Compounded Suspensions." Journal of Pharmaceutical Sciences.
United States Pharmacopeia. "Compounding Guidelines."
Khan, N., et al. (2020). "Gold nanoparticles in biomedical applications: risks and benefits." Journal of Nanobiotechnology.
Nogueira, D.R. et al. (2015). "Gold nanoparticle interaction with pharmaceuticals: stability and degradation insights." ACS Nano.
Martins, D., et al. (2018). "Photodegradation and stabilization of methylene blue in pharmaceutical solutions." European Journal of Pharmaceutics and Biopharmaceutics.
U.S. Food and Drug Administration (FDA). "Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics."
“Best product I’ve tried. Couldn’t be happier with the results.”
— Steve, Verified Buyer


★★★★★
Quality
Premium methylene blue for professionals.
Integrity
Client Services | Clear Choice Labs
Phone 📞 (909)766-1518 📲 ALSO Text-friendly
Email: Info@CCLMB.com
Clear Choice Labs© 2025. All rights reserved.
